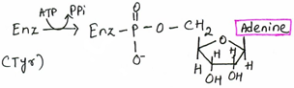Crick’s Central Dogma Genes are expressed by replication, translation and transcription of DNA. The DNA is replicated and the genetic information is translated in the form of RNA. The RNA transcripts the genetic information in the form of a proteinwhich then expresses the gene.
. 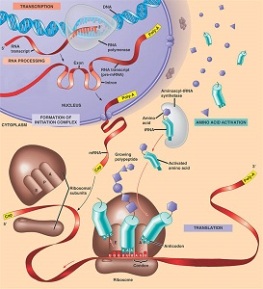
Image 107
Nucleotides are made up of a base, pentose sugar and a phosphate group. The base and the sugar make up the nucleoside. When the phosphate group is added is then a nucleotide. Nucleotides contain oxygen, carbon, phosphorus, nitrogen and hydrogen. The bases attached to the nucleotide are either pyrimidine or purines.

Image 108
Pyrimidines are thymine, uracil and cytosine. Uracil is found only in and RNA while thymine and cytosine is found in both RNA and DNA. Uracil and thymine are similar in structure so uracil replaces thymine in RNA.

Image 109
Purines are guanine and adenosine. Both are found in DNA and RNA. Erwin Chargaff discovered that there were equal ratios of adenine to thymine and guanine to cytosine in most DNA of different species. He therefore concluded that adenine pairs withthymine and guanine pairs with cytosine .

Image 110 Erwin Chargaff
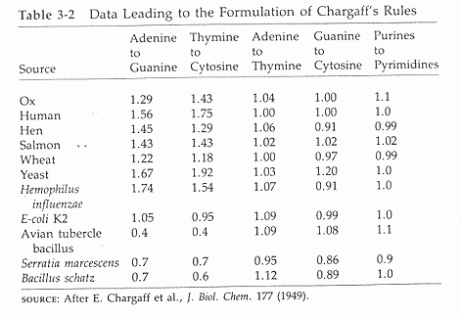
Image 111

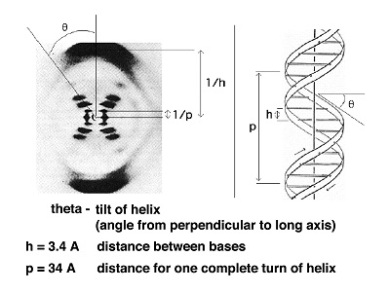
Image 112 Image 113
Rosalind Franklin discovered that DNA has a helical structure using X-ray diffraction.
James Watson and Francis Crick combined Rosalind Franklin’s X-ray data, Chargaff’s rules and examination of molecular models to discover the molecular structure of DNA. Watson and Crick proposed that if there is specific base pairing of the bases then there must be a way of replication of the genetic information. They proposed that adenine and guanine were complementary as well as cytosine and thymine because of hydrogen bonding. They also proposed that DNA is a double helix where a purine on one strand is paired to its complementary pyrimidine on the other through hydrogen bonding. The two strands are anti-parallel.

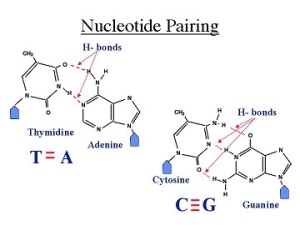
Image 114 Image 115
DNA Space Problem Since DNA is macroscopic in length, DNA is compacted or package in cells by the supercoiling of DNA. When DNA has a normal amount of base pairs per turn it is said to be relaxed. When supercoiled the helix is twisted around itself to relieve stress. Over twisting causes positive supercoiling while under twisting causes negative supercoiling. Almost all DNA in cell are circular and are negatively supercoiled.
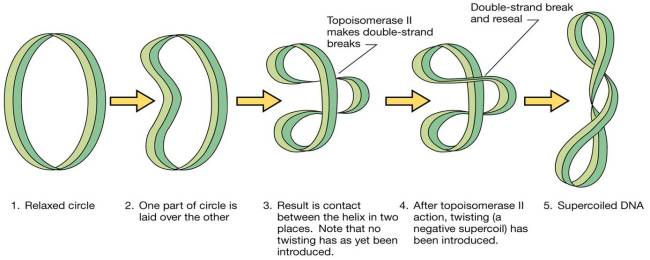
Image 116
Negative supercoiling This type of supercoiling has a higher torsional energy than relaxed DNA. This is useful for replication and transcription of DNA. Topoisomers These are DNA with the same sequence but different linking number or the number of times the strand is twisted around the central axis of the helix. Topoisomerases are a class of enzymes that regulate the amount of DNA supercoiling. Topoisomerase type 1 breaks one strand and changes the linking number in steps of ±1 while topoisomerase breaks both strands and changes the linking number in steps of ±2. Gyrase is an enzyme that causes the negative supercoiling with the aid of ATP. Nucleosides a nucleoside is a purine or pyrimidine N-glycoside bonded to a D-ribofuranose or 2-deoxy-Dribofuranose . E.g. uridine and adenosine
-. 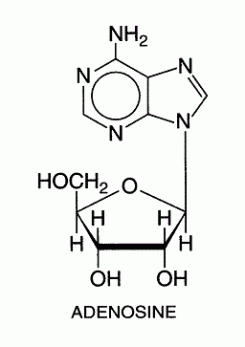
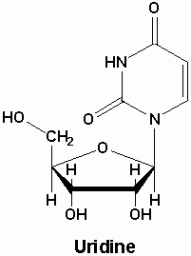
Image 117 Image 118
Nucleotides These are phosphoric esters of the nucleosides. The phosphate group is essential for nucleoside polymerisation. Nucleotides are subunits or monomers to nucleic acids such as DNA and RNA. They are used in ATP (adenosine triphosphate), as allosteric effectors and as components of enzyme cofactors e.g. NAD+.
ATP (Adenosine Triphosphate) ATP contains adenosine, ribose and a triphosphate group. It is an energy carrier of chemical potential energy. ATP releases it energy when phosphate group are removed. ATP is used in reactions such as ion transport, biosynthesis and cell movement.
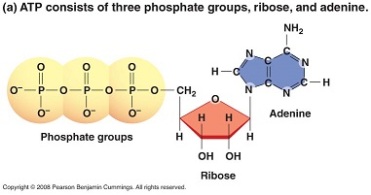
Image 119
NAD+ (Nicotinamide Adenine Diphosphate)
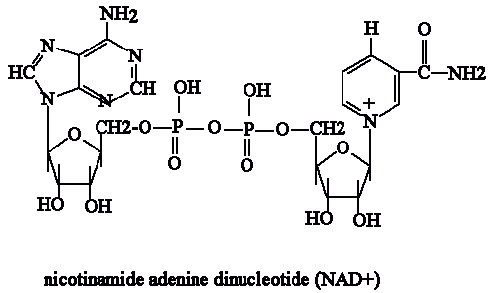
Image 120
NAD+ is a reducing agent therefore it transfers hydrogen.
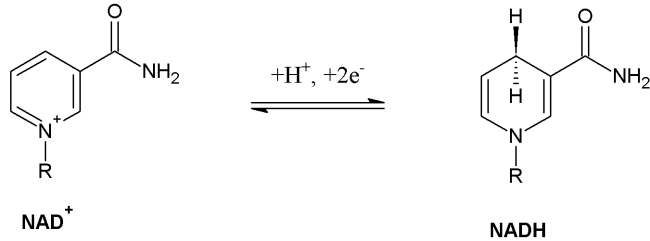
Image 121
Roles of Nucleotides Nucleotides aid in regulation of: Uridylylation ADP- ribosylation
Image 122
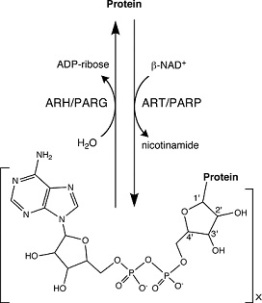
Image 123
Nucleotide and nucleoside Nomenclature 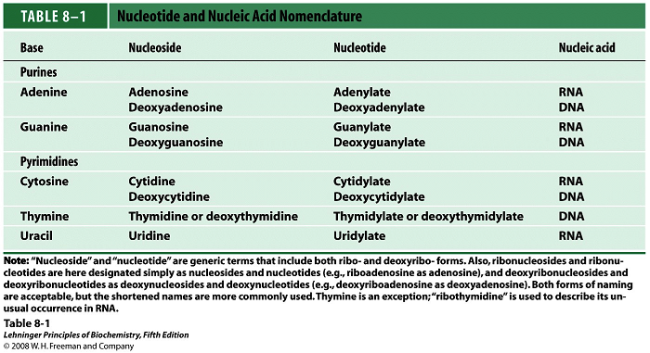
Image 124
Nucleotides in Nucleic Acids -The base is attached to the C-1 of the deoxyribose or ribose. Pyrimidine is linked in the N-1 position while purine is at the N-9 position. Nucleic Acids These are DNA and RNA and are made up of more than one nucleotide (polynucleotides).In nucleic acids the 5’ oxygen on each nucleotide is linked to the 3’ oxygen of the other. Is a double helix with the nucleotides hydrogen bonded to each other with the negatively charged sugar phosphate backbone outside with 5’ and 3’ ends. In each turn of the helix there are 10 base pairs. RNA is a single strand of polynucleotides. The nucleic acids are bonded through a phosphodiester linkage between the 3’ OH of nucleotide to the phosphate group of the other.
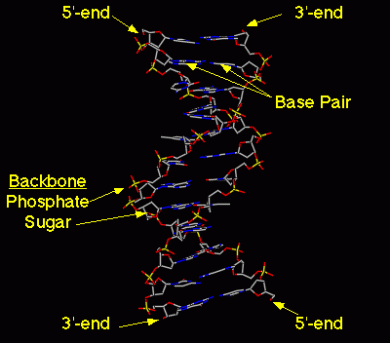
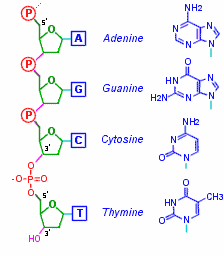
Image 125 Image 126
Roles of Nucleic Acids DNA is needed to synthesize functional genes and RNA. Regulates gene expression Ribosomal RNA (rRNA) is needed in protein synthesis. Messenger RNA (mRNA) is needed to transport the genetic material from the nucleus to the cytoplasm. Transfer RNA (tRNA) is needed is used to bring amino acids for protein synthesis.
Nucleic Acid Structures A form– favoured when little water is present. Common in RNA. B form-most common in DNA since it is the most stable conformation. Z form- formed by some DNA sequences. Structure is formed when bases are in alternating syn and anti configurations. 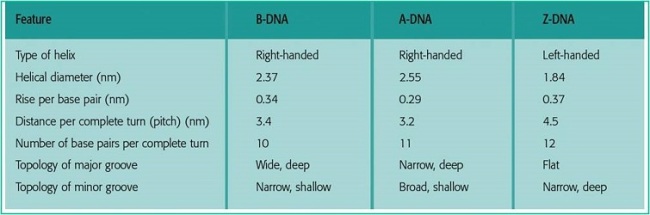
Image 127
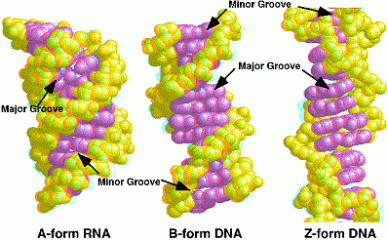
Image 128
| DNA |
RNA |
| Contains 4 bases |
Contains 4 bases |
| Has guanine, cytosine, thymine, adenine |
Has guanine, cytosine, adenine, uracil |
| Found in nucleus |
Made in nucleus works in the cytoplasm |
| Has deoxyribose sugar |
Has ribose sugar |
| Double stranded |
Single strand |
Image 129
Ribonucleic Acid (RNA) Consists of messenger RNA (mRNA), ribosomal RNA (rRNA) and transfer RNA(tRNA). mRNA transfers the genetic information from the nucleus to the cytosol. rRNA is RNA with ribosomes. These translate the genetic information from the mRNA. tRNA brings amino acids to the mRNA for protein synthesis. Stability of Nucleic Acids
Hydrogen Bonding
Due to hydrogen bonding of the base pairs the DNA has a double helix. Does not contribute to the stability of the DNA. Hydrophobic Interaction or Stacking Interaction The base pairs minimize interactions with water by stacking upon each other with the sugar phosphates on the outside to form a backbone. This is energetically stable and favoured for the DNA structure. The Effect of Acid In strong acids at high temperatures the nucleic acid is hydrolysed to its components. At a pH of 3-4 the glycosidic bonds between the base and the sugar is broken. The Effect of Alkali At a high ph >8 the bases undergo tautomerism where it is converted from the enol to the keto form or from the keto to the enol form. This can result in denaturationdue to change of structure.
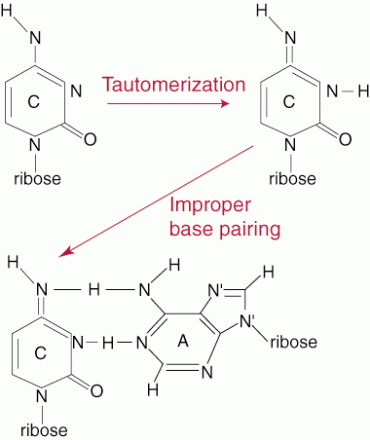
Image 130
At higher pH RNA is hydrolysed and becomes unstable. Chemical Denaturation The use of urea or formamide can destroy the hydrogen bonds and hydrophobic interactions which leads to denaturation. Buoyant Density of DNA
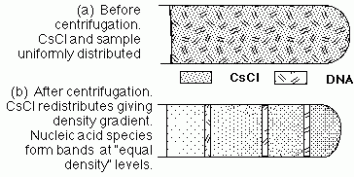
Image 131
Spectroscopic and Thermal Properties of Nucleic Acids UV absorption Bases are aromatic so can absorb light in the UV spectrum. DNA and RNA absorb a wavelength of 260nm/ A260. UV absorption is used for purity, detection and quantitation Hypochromicity due to the hydrophobic interactions of the bases to prevent interaction with water. The bases don’t absorb UV light. Quantitation of Nucleic Acids Is based on the absorption of UV light. Detects the concentration of DNA and RNA in a mixture. Beer-Lambert’s law is used to relate the amount of light absorbed to the concentration.
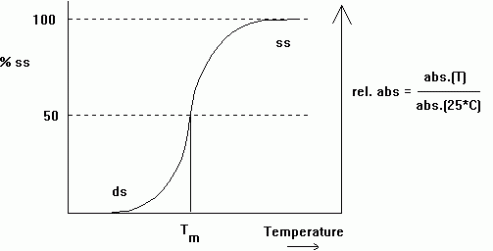
Image 132
The extinction coefficients for DNA and RNA are approximate. The value is equal to the sum of the absorbance of the different bases and depends on the amount of secondary structures as a result of Hypochromicity.

Image 133
Purity of DNA The ratio of absorbance at 260nm and 280nm is used to assess purity of DNA and RNA. A ratio of ≈1.8 is taken as pure . ≈2.0 is accepted for RNA and if the ratio is less than that it may be a protein, phenol or impurity. Thermal Denaturation Bonds are broken between the base pairs leading to denaturation. On heating the absorbance in RNA increases gradually and unevenly. The absorbance in DNA increases similarly. Renaturation When nucleic acids are cooled the absorbance decreases and the acid can reform its structure by annealing or hybridization. Annealing– base pairs of the complementary DNA are reformed due to hydrogen bonding in renaturation. Hybridization– complementary strands of the DNA reform.
References for images:
https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/central_dogma.jpg http://cs.boisestate.edu/~amit/teaching/342/lab/structure.html http://icanhas.cheezburger.com/tag/DNA http://history.nih.gov/exhibits/nirenberg/popup_htm/03_chargoff.htm https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/image7.gif https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/rosalind_franklin.jpg https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/diffract.jpg https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/article-2172894-0062e74f00000258-81_468x348.jpg https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/nucleotidepairing1.jpg https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/28.jpg https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/adenosine.gif https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/uridine.gif https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/atp02a2.jpg https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/nad.gif https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/nad_nadh.jpg https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/image_gallery.png https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/f1-large.jpg https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/nomenclature1330514034446.png https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/dna_12bp_wf.gif https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/nucleicacid.gif https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/forms-of-dna-chart.jpg https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/dnaformsabz.gif https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/copycat.jpg https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/tautomerization.gif https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/biol_213_isopynic_centrifugation.gif https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/image79.gif https://biochem1362blog.wordpress.com/wp-content/uploads/2014/04/dna-analysis-purchased-1-24-2013.png